Formation Mechanism of Manganese Dithionate for Pyrolusite Leaching with SO2
-
摘要: 副产物连二硫酸锰(MnS2O6)的生成是限制软锰矿烟气脱硫技术广泛工业化应用的关键科学问题。迄今,MnS2O6的生成机制尚未阐明,难以为控制MnS2O6的生成提供有效的理论依据。本文采用理论分析与实验验证相结合的方法研究了SO2浸出软锰矿体系MnS2O6的生成机制,阐明了MnS2O6生成速率的控制步骤和动力学过程。首先,通过文献研究对反应体系进行了理论分析,并基于SO2氧化的HSO3自由基机理及表面吸附和电化学模型提出的SO2还原浸出二氧化锰(MnO2)的动力学模型,提出MnS2O6的生成机制可用HSO3自由基生成机理进行解释,MnS2O6生成速率微观上取决于HSO3生成速率,宏观上主要取决于体系H+和
$\text{HS}{\text{O}}_{\text{3}}^{{-}} $ 浓度,理论推导的生成速率方程为${{R}}_{\text{Mn}{\text{S}}_{{2}}{\text{O}}_{{6}}}{=}{k}\cdot [{\text{H}}^{{+}}]\cdot [{\text{HSO}}_{{3}}^{{-}}]$ ,H+和$\text{HS}{\text{O}}_{\text{3}}^{{-}}$ 的理论反应级数均为1.0。然后,通过动力学实验研究考察了体系SO2浓度、pH和温度对MnS2O6生成的影响和动力学过程,结果表明:MnS2O6生成速率随体系SO2浓度的增大而增加,随体系酸度和温度的升高呈现先快速减小后下降趋缓的趋势,H+和SO2浓度对MnS2O6生成速率的反应级数分别为–0.059和1.014,反应活化能为7 068.98 J/mol。最后,结合动力学实验研究结果和SO2溶解平衡分析,推导出体系H+浓度和$\text{HS}{\text{O}}_{\text{3}}^{{-}} $ 浓度的反应级数分别为0.955和1.014,与理论推导出的反应级数非常接近。研究结果验证了理论分析所得动力学方程的准确性,表明MnS2O6生成机制可用HSO3自由基生成机理进行解释,可为MnS2O6生成特性及抑制方法研究提供理论依据和有效途径。Abstract: The formation of manganese dithionate is the key scientific issue limiting the extensive industry application of flue gas desulfurization with pyrolusite. So far, the formation mechanism of manganese dithionate has not been clarified, which is difficult to provide effective theoretical guides for controlling manganese dithionate formation. The rate-controlling steps and dynamic process of manganese dithionate formation for pyrolusite leaching process with sulfur dioxide waste gas has been clarified in this paper based on the combination of theoretical analysis and experimental verification. Firstly, the reaction system was analyzed theoretically through literature studies, based on the free radical production mechanism of SO2 oxidation and the reduction leaching kinetics model derived from surface complexation and electrochemical model were proposed. The formation mechanism of manganese dithionate can be proposed based on HSO3 free radical formation mechanism, which has manifested that the formation rate of manganese dithionate was depended on the production rate of HSO3 free radical in microscopic scales, and can be determined by H+ and$\text{HS}{\text{O}}_{\text{3}}^{{-}} $ concentration in macroscopic view. The derived theoretical equation for manganese dithionate formation could be reported as follows${{R}}_{\text{Mn}{\text{S}}_{\text{2}}{\text{O}}_{{6}}}{=}{k}\cdot{[}{\text{H}}^{{+}}]\cdot[{\text{HSO}}_{\text{3}}^{{-}}] $ , the reaction orders with respect to H+ and$\text{HS}{\text{O}}_{\text{3}}^{{-}} $ both were 1.0. Then, the effects of SO2 concentration, pH and temperature on manganese dithionate formation and dynamical processes were explored through dynamic experiments, the results showed that the manganese dithionate formation rate was decreased rapidly firstly, and then decreased easing up with pH and temperature, while raised under higher SO2 concentration conditions, the calculated apparent activation energies for manganese dithionate formation was 7 068.98 J/mol, the reaction orders with respect to H+ and SO2 concentration were –0.059 and 1.014, respectively. Finally, in combination with dynamical results and dissolution equilibrium analysis of SO 2, the reaction orders of manganese dithionate with H+ and$\text{HS}{\text{O}}_{\text{3}}^{{-}} $ were calculated as 0.955 and 1.014, respectively, which was extremely close to the theoretical reaction order of derived theoretical equation and verified the accuracy of theoretical analysis. This study showed that the formation mechanism of manganese dithionate could be explained HSO3 free radical mechanism, which could provide effective theoretical basis and approaches for ascertaining the formation characteristics of manganese dithionate and exploring corresponding inhibition methods.-
Keywords:
- sulfur dioxide /
- pyrolusite /
- manganese dithionate /
- formation mechanism /
- dynamic
-
软锰矿烟气脱硫副产硫酸锰工艺将软锰矿湿法冶金和湿法废气脱硫工艺相结合,利用SO2直接将软锰矿中的二氧化锰(MnO2)还原为硫酸锰(MnSO4)产品,不需要额外消耗还原剂和硫酸,实现了废气SO2[1-2]和低品位软锰矿[3-4]的同步资源化,对锰制品行业可持续发展有着显著的促进作用[5-7]。
目前,副产物连二硫酸锰(MnS2O6)的生成是该技术亟待解决的关键科学问题[8-10]。MnS2O6常温下极其稳定[5,11],耐氧化性强,若不能有效控制,当浸出液用来生产硫酸锰产品时,在蒸发浓缩结晶过程中大部分MnS2O6易受热分解,影响产品纯度[12-14]并产生二次污染[15-16];若浸出液用于电解锰制品生产,MnS2O6会影响电解过程和产物纯度。因此,副产物MnS2O6的生成,已成为限制该工艺在未来广泛工业化应用的技术瓶颈和关键科学问题[17-18]。
迄今,国外针对该体系中MnS2O6生成的研究几乎没有,国内也只有少量研究着眼于定性描述不同工艺条件对MnS2O6生成的影响。欧阳昌伦等[19]认为酸度、温度和含氧量是影响锰矿湿法脱硫过程中连二硫酸盐生成的主要因素,并提出SO2浓度和含氧量是通过改变体系酸度而间接产生影响,但其研究结论未得到其他学者认同。钟淦逢等[20]认为酸度、反应温度和SO2浓度是影响MnS2O6生成的主要因素。阳启华等[21]通过热力学分析和实验研究后认为,适当降低SO2/MnO2比、SO2浓度及提高体系酸度等措施,可通过调节体系pH和氧化还原电位控制MnS2O6的生成。刘晓国等[22]对MnS2O6的制备工艺进行了研究,结果显示,较低的温度和酸度、近饱和的SO2浓度和较低的SO2/MnO2比可有效促进MnS2O6的生成。然而,几乎所有关于抑制MnS2O6生成的研究都着眼于定性描述酸度、温度等工艺条件的影响,尚未能阐明MnS2O6的生成机制,难以提供有效的控制措施。
基于此,通过研究SO2浸出软锰矿体系MnS2O6的生成机制,弄清体系MnS2O6的生成过程、速率控制步骤和动力学过程,可为MnS2O6控制工艺研究提供理论支持。
1. 实验部分
1.1 实验材料
软锰矿样品产自广西桂平市,矿物平均粒径D90为4.75 μm,比表面积为8.239 m2/cm3。样品经破碎球磨处理,过200目振动筛。软锰矿化学成分分析见表1。
表 1 软锰矿化学成分质量组成Table 1 Pyrolusite compositions% Mn Fe Ca Al Mg Ni Zn Cr 32.54 4.43 1.00 1.09 0.11 0.05 0.04 0.03 1.2 实验装置
实验装置如图1所示,反应在1 L四口烧瓶中进行,正中口外接恒速搅拌装置,侧三口分别为进气口、测温口和取样口/出气口。实验过程中,反应器除通气除氧阶段外,其他时段维持密封状态,烧瓶置于恒温水浴锅中。
1.3 实验方法
实验用0.5 mol/L硫酸溶液和实验用水,配制不同酸度的溶液。将800 mL上述溶液加入烧瓶,开启搅拌(600 rpm)和水浴加热,待溶液升温至实验所需温度,立即通入气量为10 L/min的N2,持续15 min去除溶解氧;随后密封,并通过蠕动泵缓慢鼓入SO2气体,待溶液中SO2浓度达到实验所需浓度并恒定后(碘量法测定SO2浓度),停止鼓气;然后,瞬时加入过量软锰矿样品(实验时为10 g)并立即开始计时,间隔时间取样,样品经离心过滤后测定溶液中
$\mathrm{S}_2 \mathrm{O}_6^{2-} $ 浓度。软锰矿组分采用XRF(XRF–1800, SHimadzu)和ICP–MS(Nexion 300X, PE)分析;软锰矿粒径采用激光粒度仪(Hydro 2000MU(A))测定;液相 SO2浓度采用碘量法(HG/T 2967–2000)测定;
$\mathrm{S}_2 \mathrm{O}_6^{2-} $ 浓度采用离子色谱(Dionex IC–2500)测定[10]。2. MnS2O6的生成机制理论分析
SO2浸出软锰矿体系MnS2O6的生成机制尚未形成统一认识。Higginson等[23]研究了酸性条件下Fe3+氧化SO2的动力学行为,提出自由基HSO3生成机制以解释氧化产物(
$\mathrm{SO}_4^{2-} $ 和$\mathrm{S}_2 \mathrm{O}_6^{2-} $ )的生成,即:$$ {\qquad \text{Fe}\left({\text{Ⅲ}}\right)+{\text{H}}_{\text{2}}\text{S}{\text{O}}_{\text{3}}\rightarrow \text{Fe}\left({\text{Ⅱ}}\right)+\text{HS}{\text{O}}_{\text{3}} }$$ (1) $$ \text{2HS}{\text{O}}_{\text{3}} \rightarrow {\text{H}}_{\text{2}}{\text{S}}_{\text{2}}{\text{O}}_{\text{6}} $$ (2) Senanayake[24]在该自由基机制基础上,基于表面吸附[25]和电化学模型[26]提出了SO2还原浸出MnO2的动力学模型,认为MnS2O6的生成机制可能如下:
1)自由基生成:
$$ \text{pH}\leq 3 \text{时:}\text{Mn}{\text{O}}_{\text{2}}\left(\text{s}\right)+{\text{H}}^{+}+\text{HS}{\text{O}}_{\text{3}}^{-} \rightarrow \text{Mn}{\text{O}}_{\text{2}}{\text{H}}^{+}\cdot \text{HS}{\text{O}}_{\text{3}}^{-} $$ (3) $${\quad\;\; \text{Mn}{\text{O}}_{\text{2}}{\text{H}}^{+}\cdot \text{HS}{\text{O}}_{\text{3}}^{-} \rightarrow \text{MnOOH}\left(\text{s}\right)+\text{HS}{\text{O}}_{\text{3}}\text{(aq)}} $$ (4) $$\begin{aligned}[b] \text{pH}>{3}\text{时:}&\text{Mn}{\text{O}}_{\text{2}}\left(\text{s}\right)+\text{S}{\text{O}}_{{2}}\left(\text{aq}\right)+{\text{H}}_{\text{2}}\text{O} \rightarrow \\& \text{MnOOH}\left(\text{s}\right)+\text{HS}{\text{O}}_{{3}}\text{(aq)} \end{aligned}$$ (5) 2)自由基增长:
$$ {\quad \text{MnOOH}\left(\text{s}\right)+{\text{H}}^{+}+{\text{HSO}}_{\text{3}}^{-} \rightarrow \text{MnO}\left(\text{s}\right)+\text{HS}{\text{O}}_{\text{3}}\text{(aq)} }$$ (6) 3)自由基聚合:
$$ \text{2HS}{\text{O}}_{\text{3}}\left(\text{aq}\right) \rightarrow {\text{H}}_{\text{2}}{\text{S}}_{{2}}{\text{O}}_{{6}}\text{(aq)} $$ (7) $${\quad {\text{H}}_{{2}}{\text{S}}_{{2}}{\text{O}}_{{6}}\left(\text{aq}\right)+\text{MnO}\left(\text{s}\right) \rightarrow \text{Mn}{\text{S}}_{{2}}{\text{O}}_{{6}}\left(\text{aq}\right)+{\text{H}}_{{2}}\text{O}} $$ (8) 4)MnS2O6生成的总反应:
$$ {\qquad \text{Mn}{\text{O}}_{{2}}\left(\text{s}\right)+\text{2S}{\text{O}}_{{2}}\left(\text{aq}\right) \rightarrow \text{Mn}{\text{S}}_{{2}}{\text{O}}_{{6}}\left(\text{aq}\right)} $$ (9) 其中,反应式(6)是整个反应的速率控制步骤,可视为由两个电极反应组成:
$${阴极:}\text{MnOOH}\left(\text{s}\right)+{\text{H}}^{+}+{\text{e}}^{-} \rightarrow \text{MnO}\left(\text{s}\right)+{\text{H}}_{{2}}\text{O} $$ (10) $$ {\qquad {阳极:}\text{HS}{\text{O}}_{\text{3}}^{-}\left(\text{aq}\right)-{\text{e}}^{-} \rightarrow \text{HS}{\text{O}}_{{3}}\text{(aq)}} $$ (11) HSO3自由基的生成速率为:
$$ {\qquad {{R}}_{\text{HS}{\text{O}}_{{3}}}={k}\cdot {{[}{\text{H}}^{+}{]}}^{\text{0.5}}\cdot {\text{[HS}{\text{O}}_{{3}}^{-}{]}}^{{0.5}}} $$ (12) 式中:
${{R}}_{\text{HS}{\text{O}}_{{3}}}$ 为自由基HSO3的生成速率,mol/(L·s);k为反应速率常数。然而,Senanayake[24]的研究为综述性研究,是基于其他学者已有的研究成果[23,25-26],理论推导并提出SO2还原MnO2体系的混合动力学模型,推导重点在于阐明锰浸出速率和阴离子生成速率之间的关系,仅从基于速率控制步骤给出了HSO3自由基的生成速率表达式,没有确定MnS2O6的动力学表达和进行实验验证。基于此,本文对MnS2O6生成速率的具体动力学表达进行了如下推导:
根据反应式(2)或反应式(7),
$ {\text{H}}_{\text{2}}{\text{S}}_{\text{2}}{\text{O}}_{\text{6}} $ 的生成速率可表示为:$$ {{R}}_{{\text{H}}_{{2}}{\text{S}}_{{2}}{\text{O}}_{{6}}}={{[}{{R}}_{\text{HS}{\text{O}}_{{3}}}{]}}^{{2}} $$ (13) 将式(12)代入式(13),可得:
$${\qquad\quad {{R}}_{{\text{H}}_{{2}}{\text{S}}_{{2}}{\text{O}}_{{6}}}={k}\cdot{[}{\text{H}}^{+}]\cdot [{\text{HSO}}_{{3}}^{-}{]} }$$ (14) 反应式(8)为极快速反应,可认为
$ {\text{H}}_{\text{2}}{\text{S}}_{\text{2}}{\text{O}}_{\text{6}} $ 的生成速率为MnS2O6的生成速率,因此:$${\qquad \quad {{R}}_{\text{Mn}{\text{S}}_{{2}}{\text{O}}_{{6}}}={k}\cdot{[}{\text{H}}^{+}]\cdot[{\rm{HS}}{\text{O}}_{{3}}^{-}{]} }$$ (15) 综上,在SO2还原浸出MnO2过程中,产物MnS2O6的生成机制可用HSO3自由基生成机制进行解释,MnS2O6的生成速率可表达为
${{R}}_{\text{Mn}{\text{S}}_{{2}}{\text{O}}_{{6}}}{=}{k}\cdot{[}{\text{H}}^{{+}}]\cdot [{\text{HSO}}_{{3}}^{{-}}{]}$ 。为验证推导过程的准确性,本文基于SO2浸出软锰矿体系进行了针对性动力学实验研究。3. 实验结果与讨论
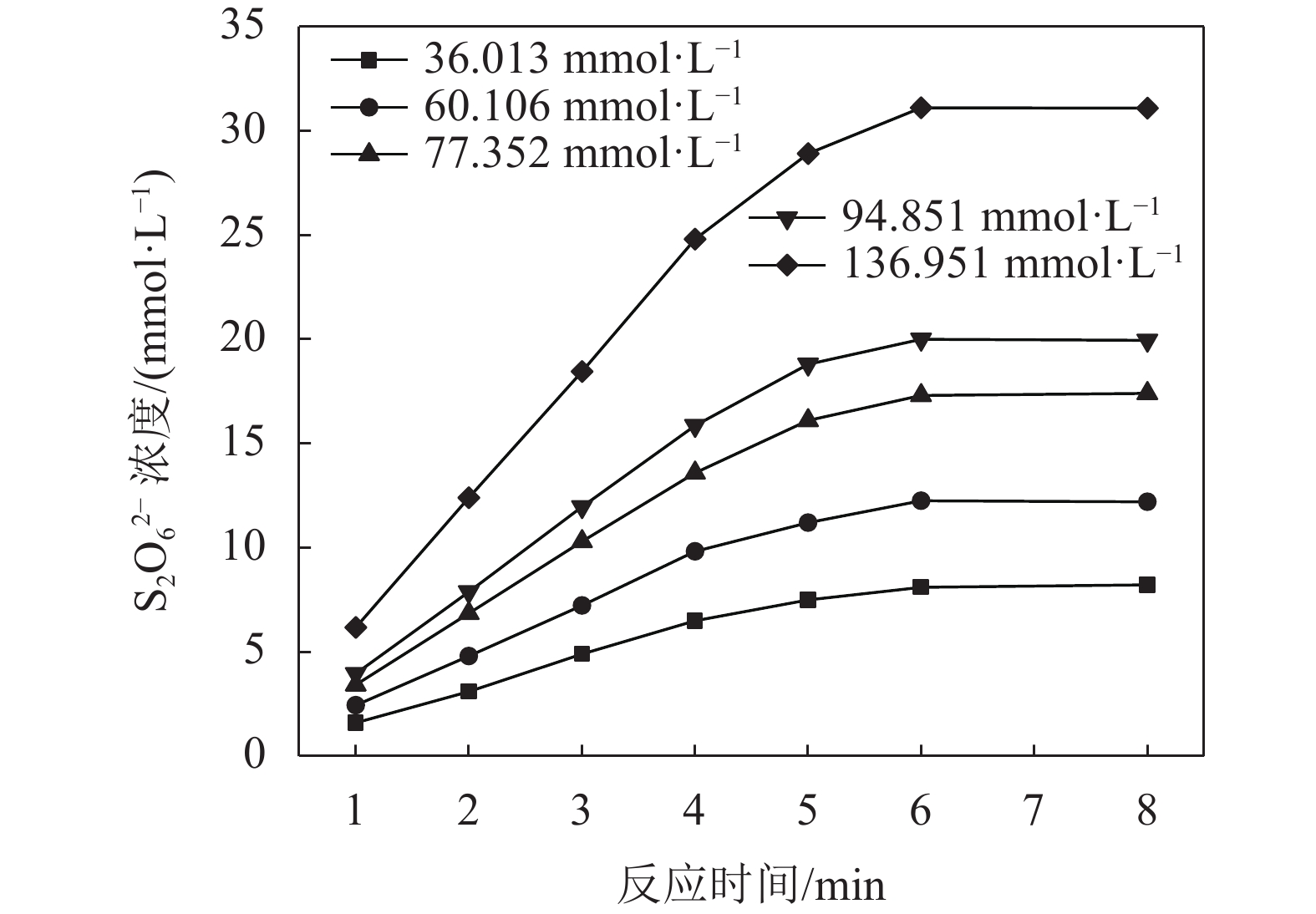
3.1 SO2浓度的影响
当温度为298.0 K,体系pH为0.70时,考察SO2浓度对MnS2O6生成速率的影响,结果如图2所示。
由图2可知:MnS2O6生成量随SO2浓度的增大而增大,随浸出时间的增长呈现出先快速增加后增势趋于平缓的变化规律;此外,浸出时间相同时,SO2浓度越高,MnS2O6生成速率也越大。
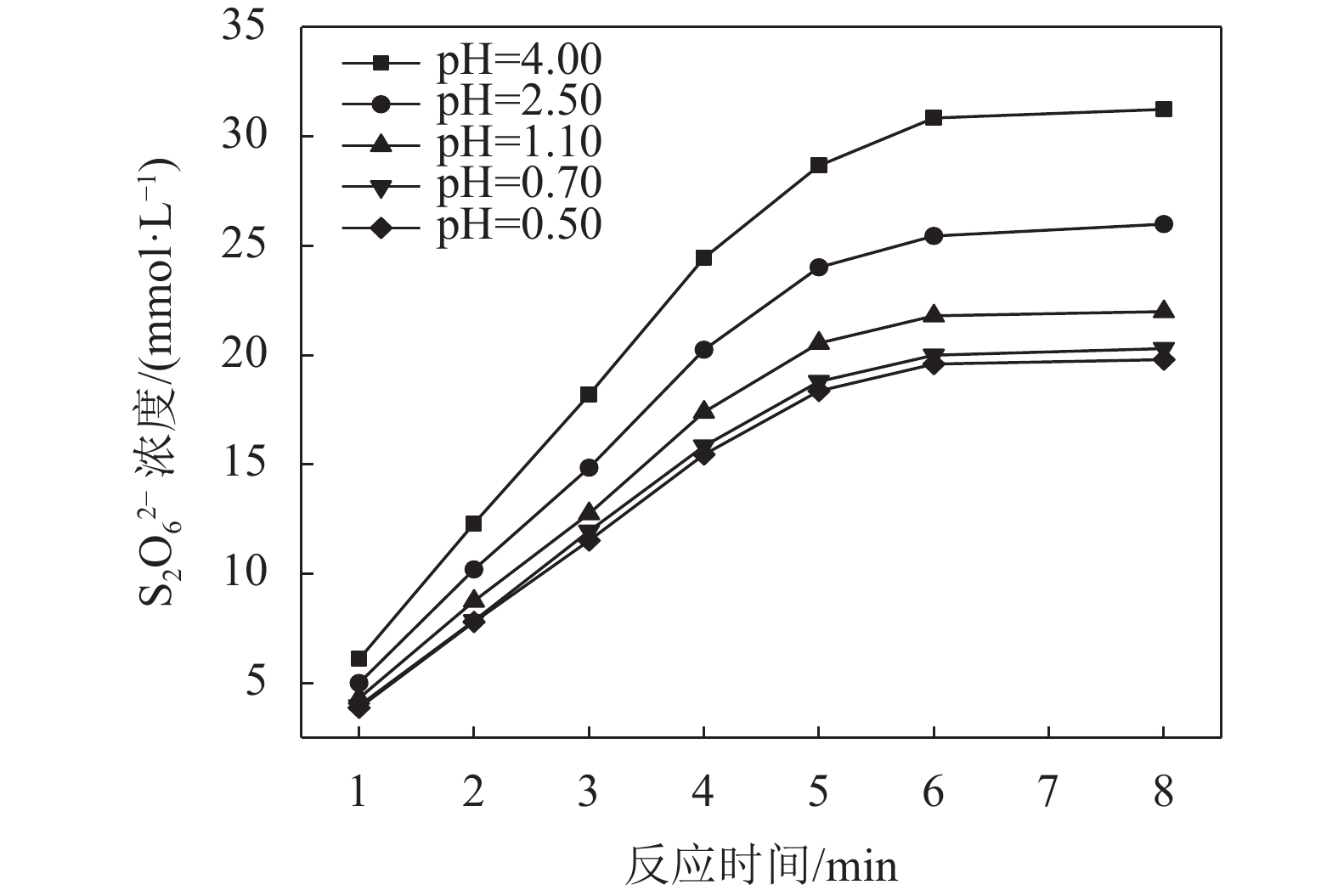
3.2 反应pH的影响
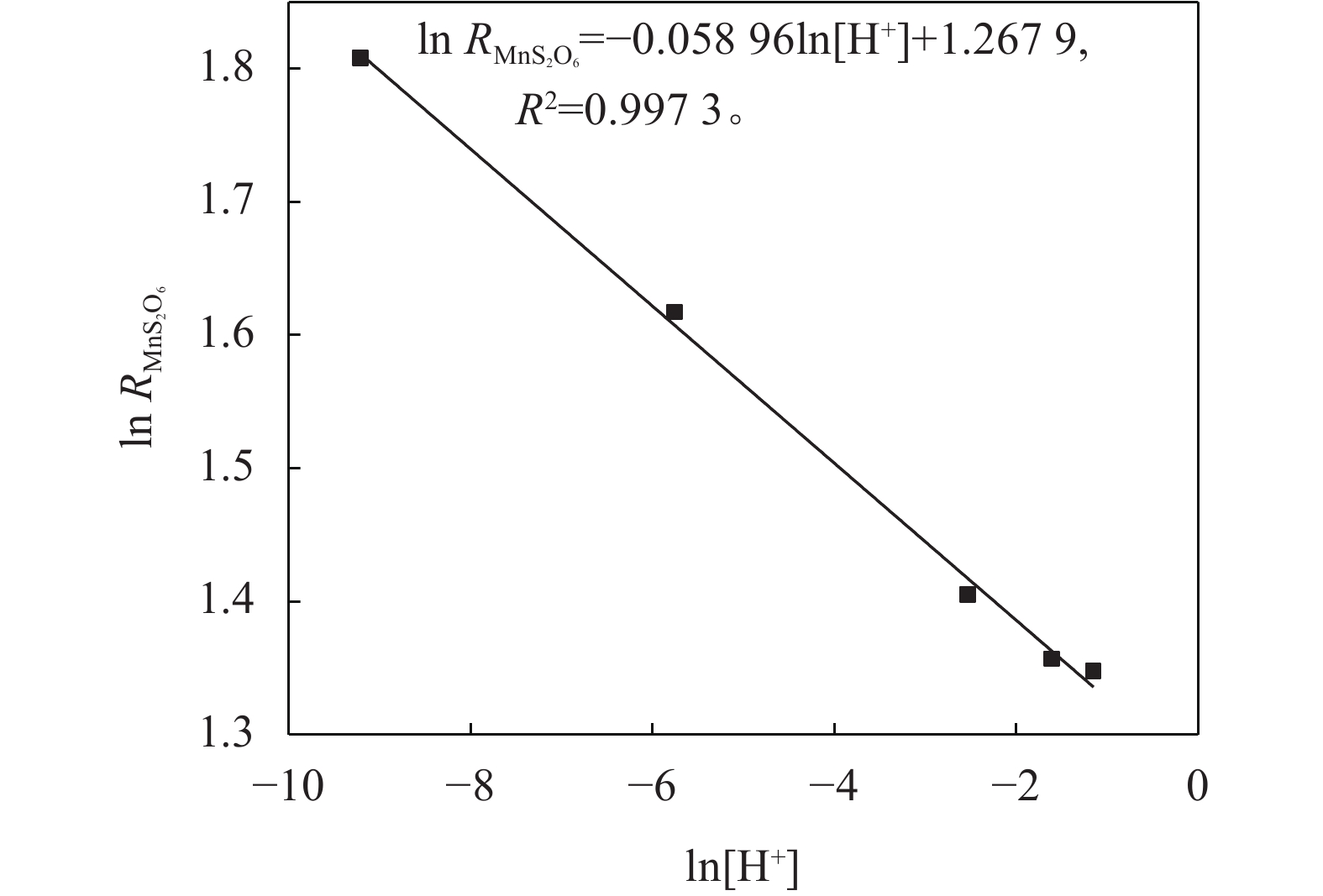
在SO2浓度为94.851 mmol/L,温度为298.0 K条件下考察pH值对MnS2O6生成速率的影响,结果如图3所示。由图3可知:MnS2O6生成速率随反应酸度的增大先快速降低后降低趋势趋缓,说明一定条件下增大酸度有利于抑制MnS2O6的生成;此外,体系pH低于0.70时,继续增大酸度,抑制显著减弱,说明酸度的影响存在一定限度。
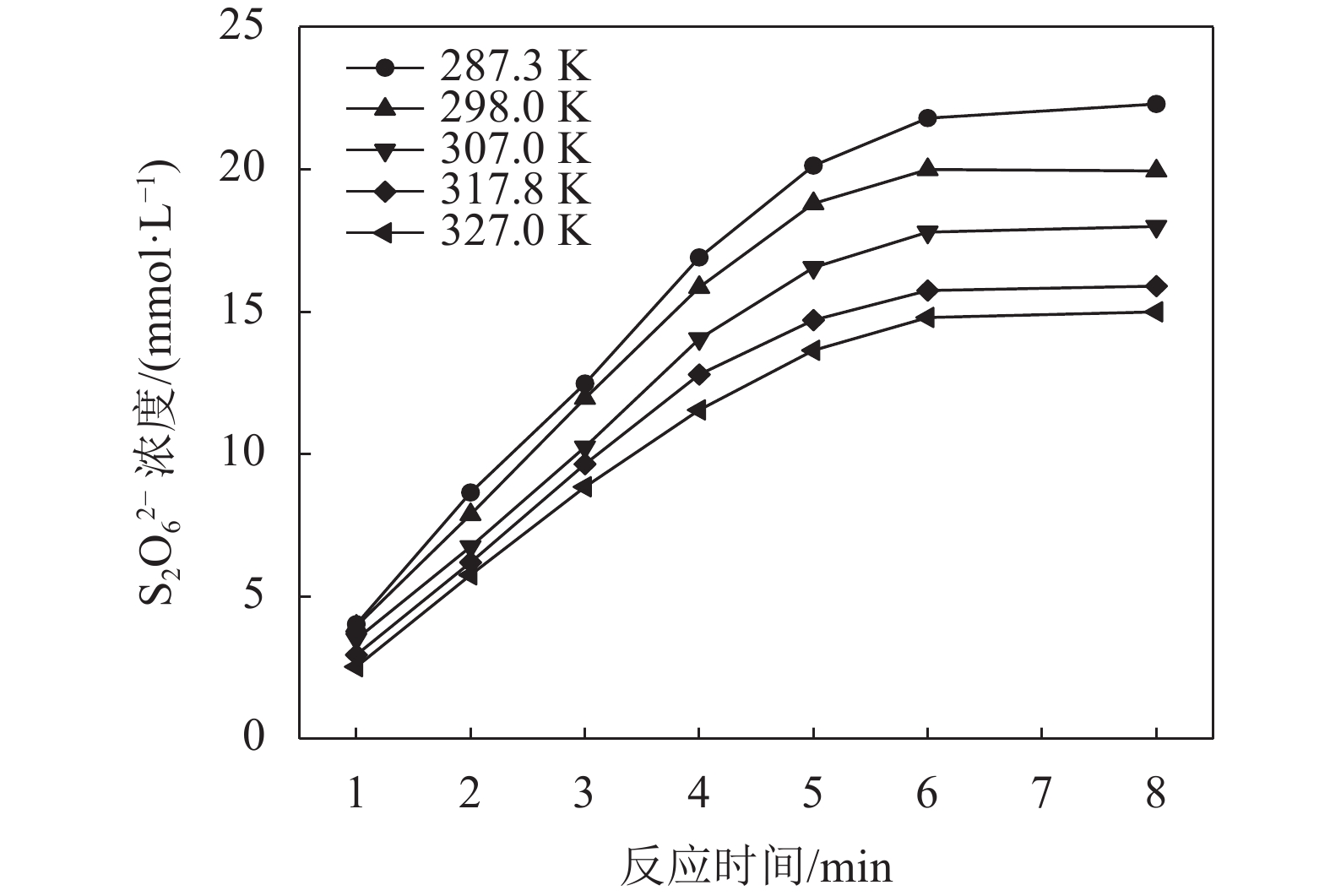
3.3 反应温度的影响
在SO2浓度为94.851 mmol/L,体系pH为0.70条件下考察反应温度对MnS2O6生成速率的影响,结果如图4所示。由图4可知:较低的温度有利于MnS2O6的生成;温度由287.3 K 升至327.0 K,反应终止时,体系中MnS2O6生成量降低了32.7%,说明提高温度有利于抑制MnS2O6的生成。
3.4 动力学分析
MnS2O6的生成动力学过程尚无可借鉴和拟合的动力学模型。基于MnS2O6的生成机制和推导的生成速率表达式,MnS2O6生成速率采用幂指数形式:
$${\qquad {{R}}_{\text{Mn}{\text{S}}_{{2}}{\text{O}}_{{6}}}={k}\cdot{{[}{\text{H}}^{+}{]}}^{{m}}\cdot{[{\text{SO}}_{{2}}{]}}^{{n}}} $$ (16) 反应速率与温度的关系利用阿伦尼乌斯公式表示:
$$ {k}={A}\cdot {\text{e}}^{E_{\text{a}}/{{RT}}} $$ (17) 对式(16)和式(17)同时取自然对数有:
$$ {\qquad \text{ln}\;{{R}}_{\text{Mn}{\text{S}}_{\text{2}}{\text{O}}_{\text{6}}}=\text{ln}\;{k}+{m}\text{ln}\left[{\text{H}}^{+}\right]+{n}\text{ln[S}{\text{O}}_{\text{2}}{]} }$$ (18) $$ \text{ln}\;{k}=\text{ln}\;{A}-\frac{{{E}}_{\text{a}}}{{RT}} $$ (19) 式中:
${{R}}_{\text{Mn}{\text{S}}_{\text{2}}{\text{O}}_{\text{6}}}$ 为MnS2O6的生成速率,mmol/(L·s);m、n为反应级数;k为反应速率常数;Ea为活化能,kJ/mol;A为指前因子;R为通用气体常数,8.314;T为反应温度,K。3.4.1 SO2和H+反应级数确定
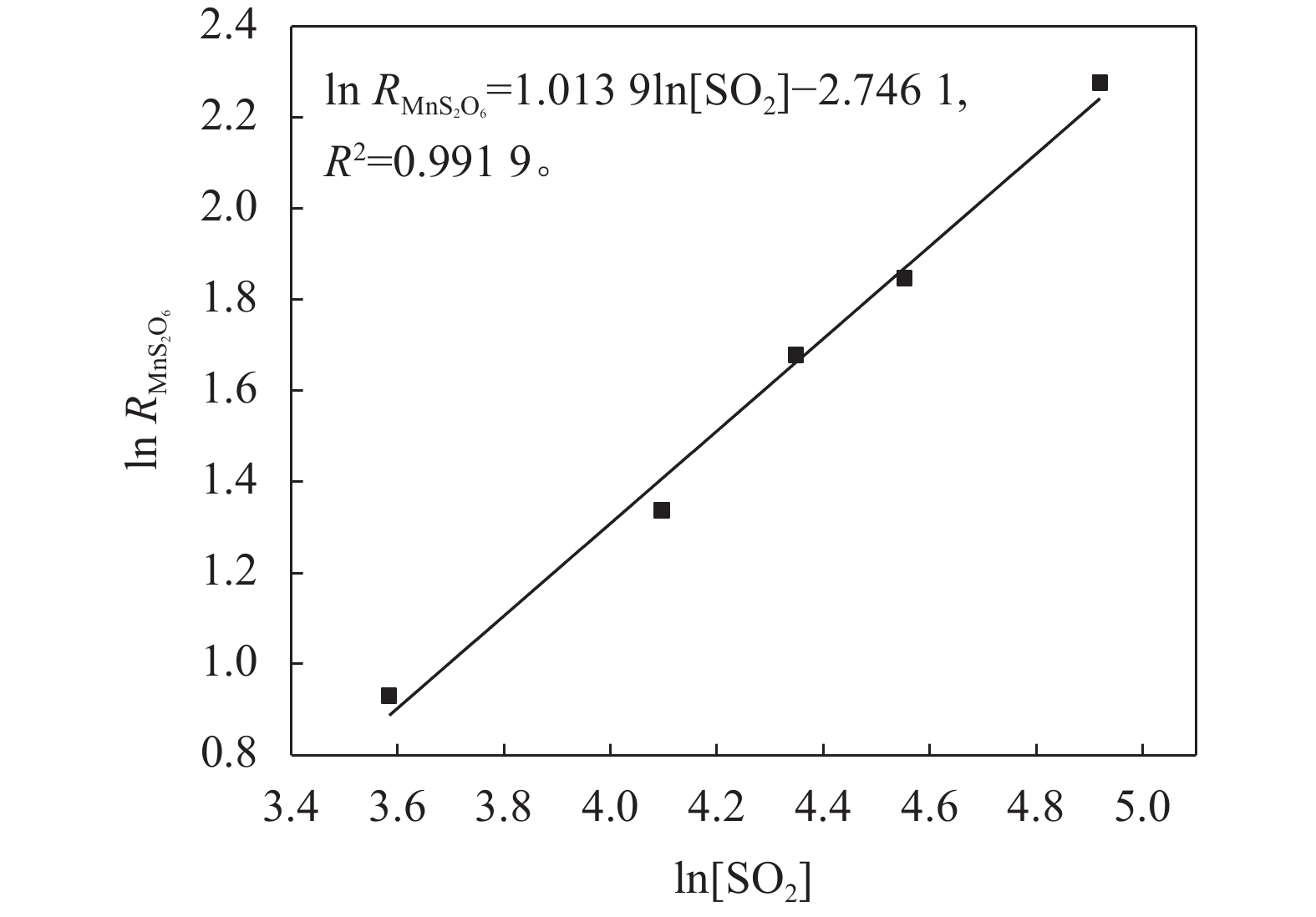
对图2和3中各时间点的生成速率进行2阶多项式拟合(y=at2+bt+c),并对拟合曲线求1阶导数,令t=0,可得到反应起点处的切线斜率,即为MnS2O6的生成速率。根据式(18)可知,分别以
$\text{ln}\;{{R}}_{\text{Mn}{\text{S}}_{\text{2}}{\text{O}}_{\text{6}}}$ 对ln[SO2]和ln[H+]进行线性拟合,拟合曲线的斜率即为SO2和H+的反应级数,结果如图5和6所示。由图图5~6可知,MnS2O6生成速率对SO2的反应级数为1.014,对体系H+浓度的反应级数为–0.059。
3.4.2 反应活化能确定
同理,基于图4进行2阶多项式拟合,可求出不同温度下MnS2O6的生成速率
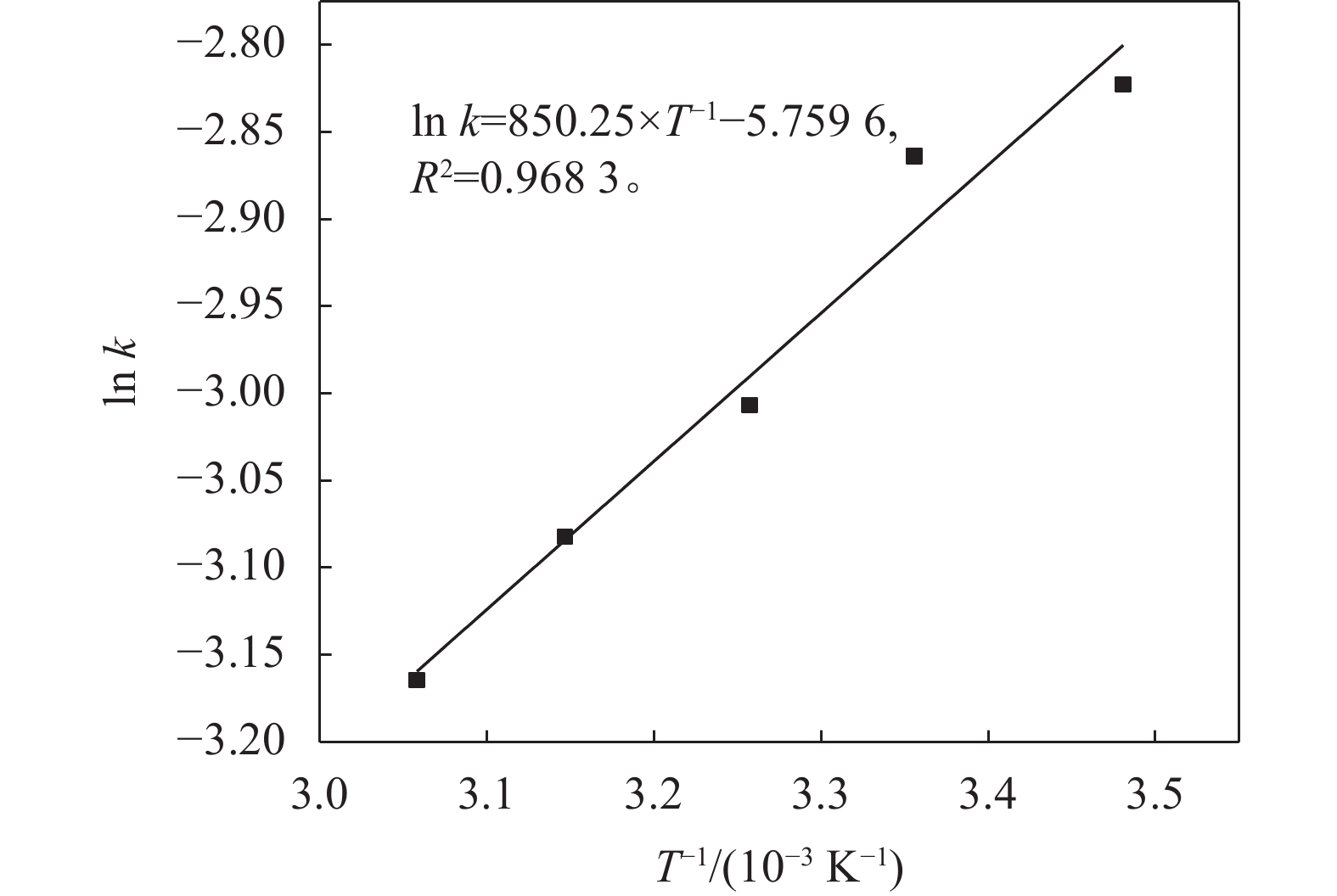
$ {{R}}_{\text{Mn}{\text{S}}_{\text{2}}{\text{O}}_{\text{6}}} $ 。此时,ln[SO2]和ln[H+]大小分别为4.5523和–1.6118,连同已求解SO2和H+的反应级数,代入式(18),可求出不同温度下的反应的平衡常数k。以ln k为纵坐标对1/T进行线性拟合,求解出反应活化能,结果如图7所示。由图7可知,反应活化能Ea为7 068.98 J/mol,指前因子为317.22 s–1。3.4.3 MnS2O6的生成动力学方程分析
综上动力学分析,MnS2O6的生成动力学可表述如下:
$$ {\quad {{R}}_{\text{Mn}{\text{S}}_{{2}}{\text{O}}_{{6}}}={317.22}{\text{e}}^{-\frac{{7\;068.98}}{{RT}}}\cdot{{[}{\text{H}}^{+}{]}}^{{-0.059}}\cdot{\text{[S}{\text{O}}_{{2}}{]}}^{{1.014}}} $$ (20) SO2溶解水中快速达到一级解离平衡[25]:
$$ {\qquad \text{S}{\text{O}}_{{2}}\left(\text{aq}\right)+{\text{H}}_{{2}}\text{O} \rightarrow {\text{H}}_{\text{2}}\text{S}{\text{O}}_{{3}}\left(\text{aq}\right)} $$ (21) $$ {\qquad {\text{H}}_{{2}}\text{S}{\text{O}}_{{3}}\left(\text{aq}\right) \rightarrow {\text{H}}^{+}+\text{HS}{\text{O}}_{{3}}^{-}} $$ (22) 解离平衡常数K1为:
$$ {\qquad {{K}}_{\text{1}}=\frac{\left[{\text{H}}^{+}\right]\cdot\text{[HS}{\text{O}}_{{3}}^{-}{]}}{{[}{\text{H}}_{{2}}\text{S}{\text{O}}_{{3}}{]}}{=0.017}} $$ (23) 将反应式(22)代入式(20)中,可知MnS2O6的生成动力学也可表示如下:
$$ {\;\; {{R}}_{\text{Mn}{\text{S}}_{{2}}{\text{O}}_{{6}}}{=1\;912.9}{\text{e}}^{-\frac{{7\;068.98}}{{RT}}}\cdot{{[}{\text{H}}^{+}{]}}^{{0.955}}\cdot{\text{[HS}{\text{O}}_{{3}}^{-}{]}}^{{1.014}} }$$ (24) 由MnS2O6的生成动力学方程可知,MnS2O6生成速率随温度和酸度的升高而减小,随液相SO2浓度的升高而增加。
4. 结 论
采用理论分析与实验验证相结合的方法研究了SO2浸出软锰矿体系MnS2O6的生成机制,阐明了MnS2O6生成速率的控制步骤和动力学过程。具体实验结论如下:
1)基于SO2氧化的HSO3自由基机理及表面吸附和电化学模型提出的SO2还原浸出MnO2的动力学模型,提出MnS2O6的生成机制可用HSO3自由基生成机理进行解释;MnS2O6的理论生成速率方程为
${{R}}_{\text{Mn}{\text{S}}_{{2}}{\text{O}}_{{6}}}{=} {k}\cdot{[}{\text{H}}^{{+}}]\cdot[{\rm{HS}}{\text{O}}_{{3}}^{{-}}]$ ,即宏观上主要取决于体系H+和${\rm{HS}}{\text{O}}_{{3}}^{{-}}$ 浓度, H+和${\rm{HS}}{\text{O}}_{{3}}^{{-}}$ 的理论反应级数均为1.0。2)MnS2O6生成动力学研究表明,MnS2O6生成速率随SO2浓度的升高而升高,随体系酸度和温度的升高先快速下降,后下降趋势趋缓。H+和SO2浓度对MnS2O6生成速率的反应级数分别为–0.059和1.014,反应活化能为7 068.98 J/mol。继而,结合SO2溶解平衡分析,推导出体系H+浓度和
${\rm{HS}}{\text{O}}_{{3}}^{{-}} $ 浓度的反应级数分别为0.955和1.014,与理论反应级数非常接近。研究结果验证了理论分析所得动力学方程的准确性,表明MnS2O6生成机制可用HSO3自由基生成机理进行解释,可为MnS2O6生成特性及抑制方法研究提供理论依据和有效途径。 -
表 1 软锰矿化学成分质量组成
Table 1 Pyrolusite compositions
% Mn Fe Ca Al Mg Ni Zn Cr 32.54 4.43 1.00 1.09 0.11 0.05 0.04 0.03 -
[1] 孙维义,丁桑岚,苏仕军,等.二氧化硫液相浸取低品位软锰矿的动力学[J].四川大学学报(工程科学版),2011,43(增刊1):199–203. doi: 10.15961/j.jsuese.2011.s1.018 Sun Weiyi,Ding Sanglan,Su Shijun,et al.Leaching kinetics of Mn from low grade pyrolusite with SO2 in liquid phase[J].Journal of Sichuan University(Engineering Science Edition),2011,43(Supp1):199–203 doi: 10.15961/j.jsuese.2011.s1.018 [2] Su Shijun,Zhu Xiaofan,Liu Yongjun,et al.A pilot-scale jet bubbling reactor for wet flue gas desulfurization with pyrolusite[J].Journal of Environmental Sciences(China),2005,17(5):827–831. [3] He Kejie,Sun Weiyi,Ding Sanglan,et al.Low pollution technology of Mn3O4 preparation by thermal decomposition of MnSO4[J].Fresenius Environmental Bulletin,2015,24(10B):3420–3425. doi: 10.1007/bf01912932 [4] Deng Lin,Qu Bing,Su Shijun,et al.Study on separation of Manganese from iron in high-iron pyrolusite ore by leaching with simulated flue gas[J].Journal of Chemical Engineering of Japan,2017,50(12):892–899. doi: 10.1252/jcej.16we377 [5] 孙维义,苏仕军,丁桑岚,等.软锰矿浆烟气同步脱硫脱硝尾液中连二硫酸锰分解特性的研究[J].四川大学学报(工程科学版),2011,43(3):166–170. doi: 10.15961/j.jsuese.2011.03.017 Sun Weiyi,Su Shijun,Ding Sanglan,et al.Decomposition characteristics of Manganese dithionate in absorption solution of flue gas desulfurization and denitration with pyrolusite slurry[J].Journal of Sichuan University(Engineering Science Edition),2011,43(3):166–170 doi: 10.15961/j.jsuese.2011.03.017 [6] 孙维义,苏仕军,丁桑岚,等.烟气氧硫比对软锰矿浆烟气脱硫体系浸锰过程及脱硫产物的影响[J].高校化学工程学报,2011,25(1):143–148. doi: 10.3969/j.issn.1003-9015.2011.01.025 Sun Weiyi,Su Shijun,Ding Sanglan,et al.Effect of O2/SO2 ratio in the flue gas on Manganese extract process and byproduct in the process of flue gas desulfurization with pyrolusite pulp[J].Journal of Chemical Engineering of Chinese Universities,2011,25(1):143–148 doi: 10.3969/j.issn.1003-9015.2011.01.025 [7] Sun Weiyi,Su Shijun,Wang Qingyuan,et al.Lab-scale circulation process of electrolytic Manganese production with low-grade pyrolusite leaching by SO2[J].Hydrometallurgy,2013,133:118–125. doi: 10.1016/j.hydromet.2012.12.005 [8] He Kejie,Su Shijun,Ding Sanglan,et al.Oxidation of SO2 by O2 and its effects on dithionate formation during pyrolusite leaching process with mixture gas containing SO2 and O2[J].ChemistrySelect,2018,3(46):13154–13160. doi: 10.1002/slct.201801894 [9] He Kejie,Su Shijun,Ding Sanglan,et al.Formation characteristics of dithionate and sulfate ions in the pyrolusite leaching process with SO2[J].Reaction Kinetics,Mechanisms and Catalysis,2018,123(2):757–770. doi: 10.1007/s11144-018-1365-5 [10] Qu Bing,Hu Wenli,Deng Lin,et al.Simultaneous determination of dithionate and sulfate in leaching solution from SO2-leaching pyrolusite by ion chromatography[J].Energy & Fuels,2016,30(10):8561–8566. doi: 10.1021/acs.energyfuels.6b01333 [11] Lente G,Fábián I.Effect of dissolved oxygen on the oxidation of dithionate ion.Extremely unusual kinetic traces[J].Inorganic Chemistry,2004,43(13):4019–4025. doi: 10.1021/ic0499087 [12] 曲兵,康禄华,李忆雯,等.连二硫酸锰碳化干渣热解回收硫酸钠的研究[J].工程科学与技术,2020,52(1):168–174. doi: 10.15961/j.jsuese.201801099 Qu Bing,Kang Luhua,Li Yiwen,et al.Study on thermal decomposition mechanisms and recovery of sodium sulfate from Manganese dithionate after carbonization[J].Advanced Engineering Sciences,2020,52(1):168–174 doi: 10.15961/j.jsuese.201801099 [13] 丁林,杨林,王成,等.二氧化锰矿分解连二硫酸锰及动力学[J].中国有色金属学报,2020,30(10):2475–2481. doi: 10.11817/j.ysxb.1004.0609.2020-39554 Ding Lin,Yang Lin,Wang Cheng,et al.Decomposition of manganous dithionate with natural MnO2 ore and kinetics study[J].The Chinese Journal of Nonferrous Metals,2020,30(10):2475–2481 doi: 10.11817/j.ysxb.1004.0609.2020-39554 [14] You Zhixiong,Li Guanghui,Zhang Yuanbo,et al.Extraction of Manganese from iron rich MnO2 ores via selective sulfation roasting with SO2 followed by water leaching[J].Hydrometallurgy,2015,156:225–231. doi: 10.1016/j.hydromet.2015.05.017 [15] 陈建伟,童张法,陈志传,等.粗MnSO4溶液中MnS2O6的去除条件研究[J].环境工程学报,2011,5(1):175–178. Chen Jianwei,Tong Zhangfa,Chen Zhichuan,et al.Study on conditions for removing MnS2O6 from MnSO4 solution[J].Chinese Journal of Environmental Engineering,2011,5(1):175–178 [16] Yang Lin,Wang Cheng,Yao Lu,et al.Removal of manganous dithionate(MnS2O6) with MnO2 from the desulfurization Manganese slurry[J].RSC Advances,2020,10(3):1430–1438. doi: 10.1039/c9ra09810k [17] Qu Bing,Deng Lin,Deng Biao,et al.Oxidation kinetics of dithionate compound in the leaching process of Manganese dioxide with Manganese dithionate[J].Reaction Kinetics,Mechanisms and Catalysis,2018,123(2):743–755. doi: 10.1007/s11144-017-1284-x [18] Qu Bing,Deng Lin,Zhang Xiaohan,et al.Enhanced recovery of dithionate from desulfurization with pyrolusite system by Manganese dioxide anode of spent batteries[J].Advanced Powder Technology,2020,31(5):2035–2044. doi: 10.1016/j.apt.2020.02.036 [19] 欧阳昌伦,谢兰香.锰矿湿法脱硫过程中影响连二硫酸盐生成的主要因素[J].广西化工,1983,12(3):60–67. Ouyang Changlun,Xie Lanxiang.Main factors affecting the formation of dithionite during wet desulfurization of Manganese ore[J].Technology & Development of Chemical Industry,1983,12(3):60–67 [20] 钟淦逢,陈洁茹.用软锰矿浆吸收SO2过程中MnS2O6的抑制和清除研究[J].广东化工,1997,24(2):28–29. Zhong Ganfeng,Chen Jieru.Study on the inhibition and removal of MnS2O6 in the process of absorbing SO2 with pyrolusite pulp[J].Guangdong Chemical Industry,1997,24(2):28–29 [21] 阳启华,张昭.SO2浸出软锰矿过程中抑制连二硫酸锰生成的研究[J].湿法冶金,2012,31(3):144–148. doi: 10.13355/j.cnki.sfyj.2012.03.011 Yang Qihua,Zhang Zhao.Study on restrain generation of Manganese dithionate in leaching of MnO2 with SO2[J].Hydrometallurgy of China,2012,31(3):144–148 doi: 10.13355/j.cnki.sfyj.2012.03.011 [22] 刘晓国,王继红,郑成,等.连二硫酸锰制备工艺和提纯方法的研究[J].广州化工,1999,27(4):37–40. Liu Xiaoguo,Wang Jihong,Zheng Cheng,et al.The study on the preparation and purification of MnS2O6[J].Guang Zhou Chemical Industry and Technology,1999,27(4):37–40 [23] Higginson W C E,Marshall J W.82.Equivalence changes in oxidation–reduction reactions in solution:Some aspects of the oxidation of sulphurous acid[J].Journal of the Chemical Society(Resumed),1957(0):447–458. doi: 10.1039/JR9570000447 [24] Senanayake G.A mixed surface reaction kinetic model for the reductive leaching of Manganese dioxide with acidic sulfur dioxide[J].Hydrometallurgy,2004,73(3/4):215–224. doi: 10.1016/j.hydromet.2003.10.010 [25] Miller J D,Wan Rongyu.Reaction kinetics for the leaching of MnO2 by sulfur dioxide[J].Hydrometallurgy,1983,10(2):219–242. doi: 10.1016/0304-386X(83)90007-5 [26] Nicol M J,Lázaro I.The role of EH measurements in the interpretation of the kinetics and mechanisms of the oxidation and leaching of sulphide minerals[J].Hydrometallurgy,2002,63(1):15–22. doi: 10.1016/S0304-386X(01)00206-7







 下载:
下载: